IONISATION POTENTIAL:- The ionisation potential is the amount of energy required to remove a valence electron from the isolated gaseous atom.
Factors affecting the ionisation potential:-
1) Atomic size:- Atomic size increases potential ionisation decreases.
2)Nuclear Charge:- Nuclear Charge increases potential ionisation increases.
TREND OF IONISATION POTENTIAL IN PERIODIC TABLE:-
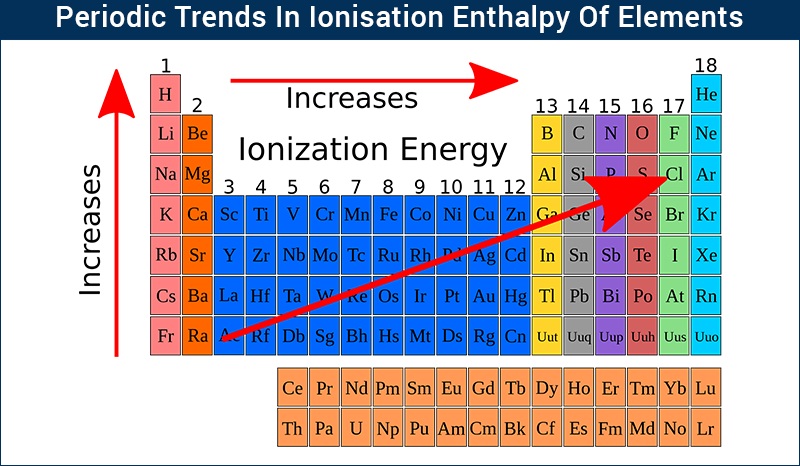
In the period:- In the period from left to right ionisation potential increases because
1) Nuclear Charge increases from left to right in the period.
2)Atomic size decreases
3)Hence In the period from left to right energy required to remove the valence electrons from an atom increases.
In the group: -from top to bottom ionisation potential of elements decreases because-
1)atomic size goes on increases from top to bottom in the same group.
2)Hence attraction force between the nucleus and valence electron decreases.
3) Thus lesser energy is sufficient to remove the valence electrons from an atom.


 0
0

